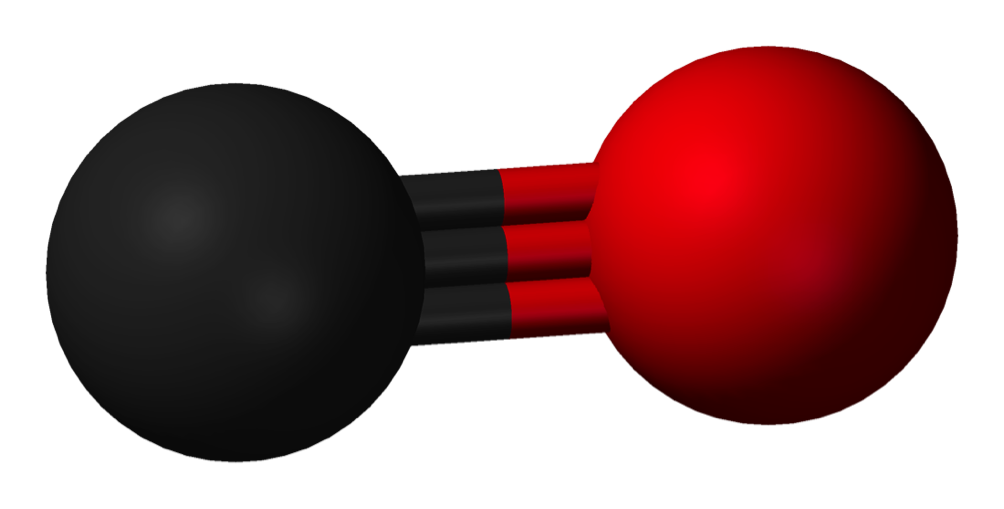
Carbon Monoxide (Co) Gas, Properties, Alarm Levels and Gas Detection
The federal Centers for Disease Control and Prevention uses just 13 words to summarize carbon monoxide. "Carbon monoxide, or 'CO,' is an odorless, colorless gas that can kill you.

CO Lewis Structure, Geometry, and Hybridization Techiescientist
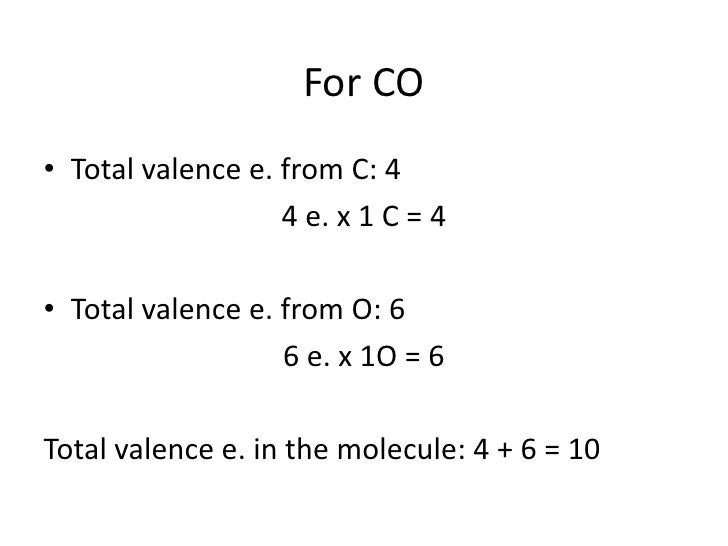
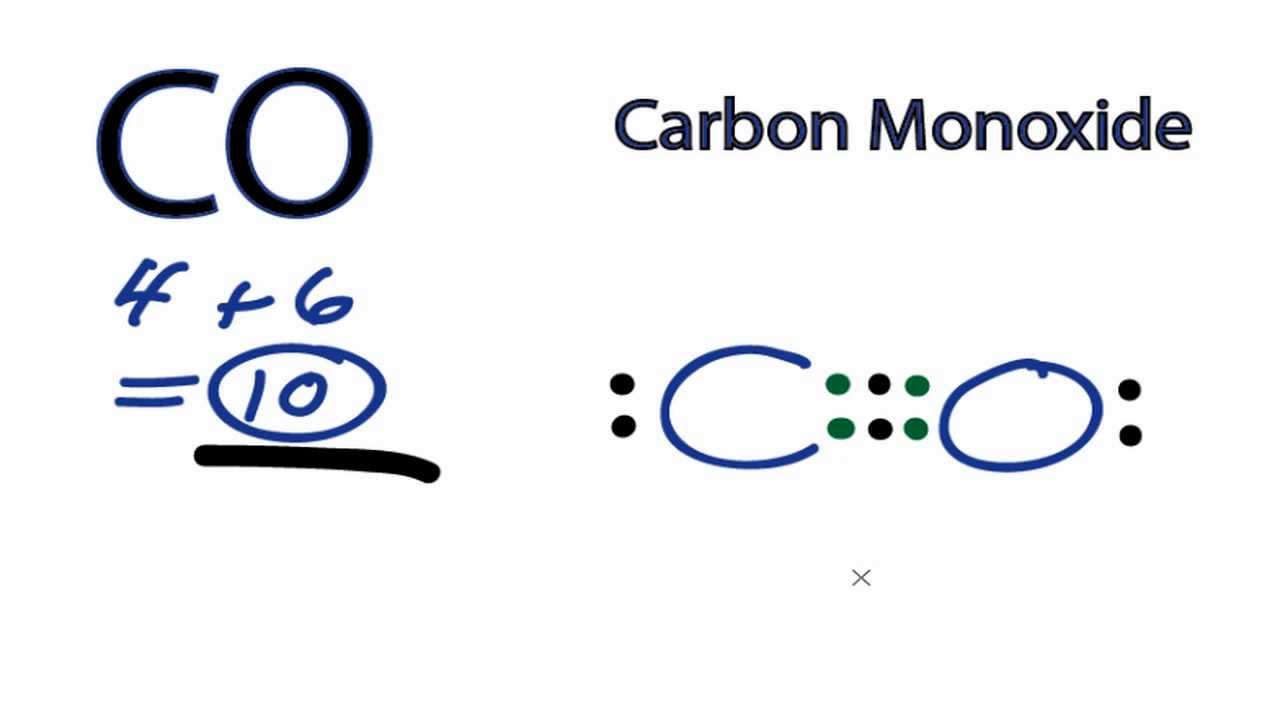
After determining how many valence electrons there are in CO, place them around the central atom to complete the octets. The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence.
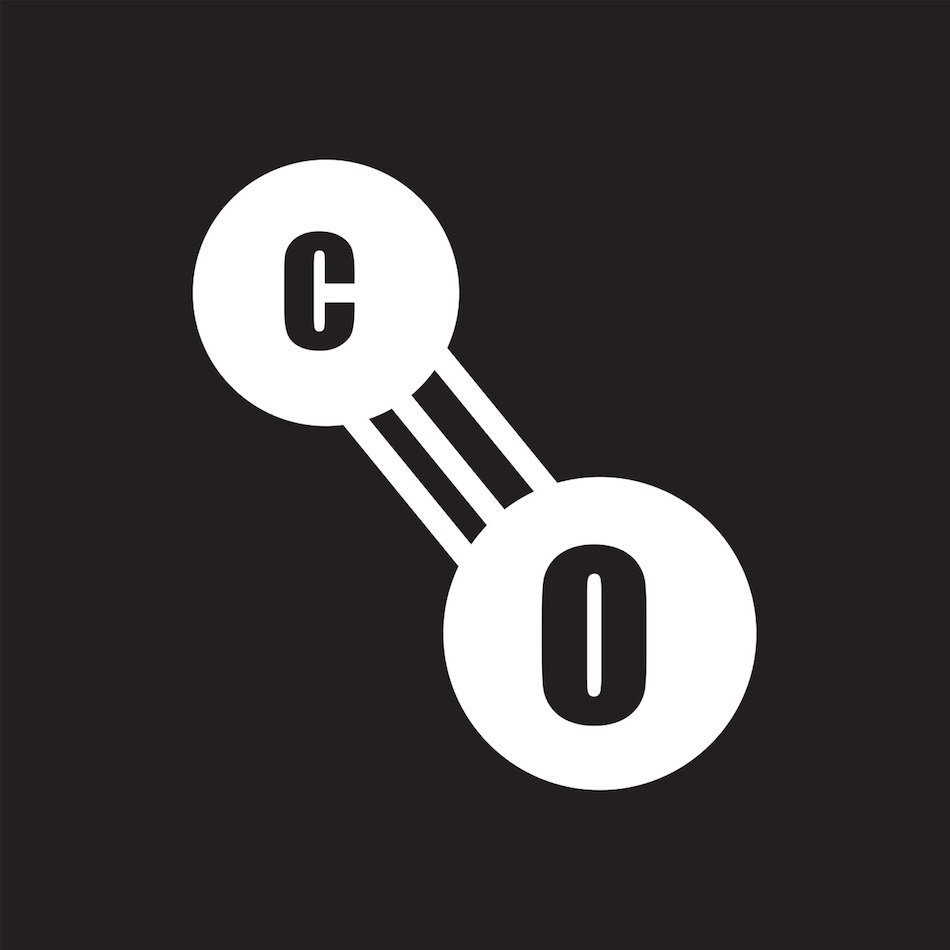
Carbon Monoxide Safety How to Protect Your Home and Respond to Emergencies
Lewis Structure of CO (Carbon Monoxide) chemistNATE 260K subscribers Subscribe Subscribed 4.6K 622K views 10 years ago Lewis Structures The Lewis Structure (Lewis Dot Diagram) for CO. 1..
Lewis Dot Structure Search Results Calendar 2015
The data on carbon monoxide indicates that the C-O distance is about 1.13 A (1 A = 10-10 m). In carbon dioxide, CO 2, the distance is 1.16 A. The shorter distance in carbon monoxide suggests the carbon and oxygen are held a little more tightly together than they are in carbon dioxide, in which the Lewis structure shows only a double bond.
40+ Carbon Monoxide Lewis Structure PNG Bepe Enthusiastic
Learning Objectives By the end of this section, you will be able to: Write Lewis symbols for neutral atoms and ions Draw Lewis structures depicting the bonding in simple molecules Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions.

CO Lewis StructureThis post is about carbon monoxide lewis structure
The carbon monoxide is produced from the partial oxidation of carbon dioxide (CO2) or any other carbon-containing element. The Lewis structure, also called as electron dot structure, is a simplified method of representing the number of valence electrons present within an atom or a molecule.

Carbon Monoxide Structure Co Lewis Structure Hybridization And
A step-by-step explanation of how to draw the CO Lewis Dot Structure (Carbon dioxide). For the CO structure use the periodic table to find the total number of valence electrons for t.more.
Carbon Monoxide Lewis Structure 10 1 Lewis Structures And The Octet
Do not use a charcoal grill indoors and do not use a gas stove for heat - they could give off harmful levels of carbon monoxide. Stay warm by dressing in layers and minimizing time spent outdoors. Be aware of cold stress symptoms (i.e., hypothermia) and seek proper medical attention if symptoms appear. Close off rooms you do not need.
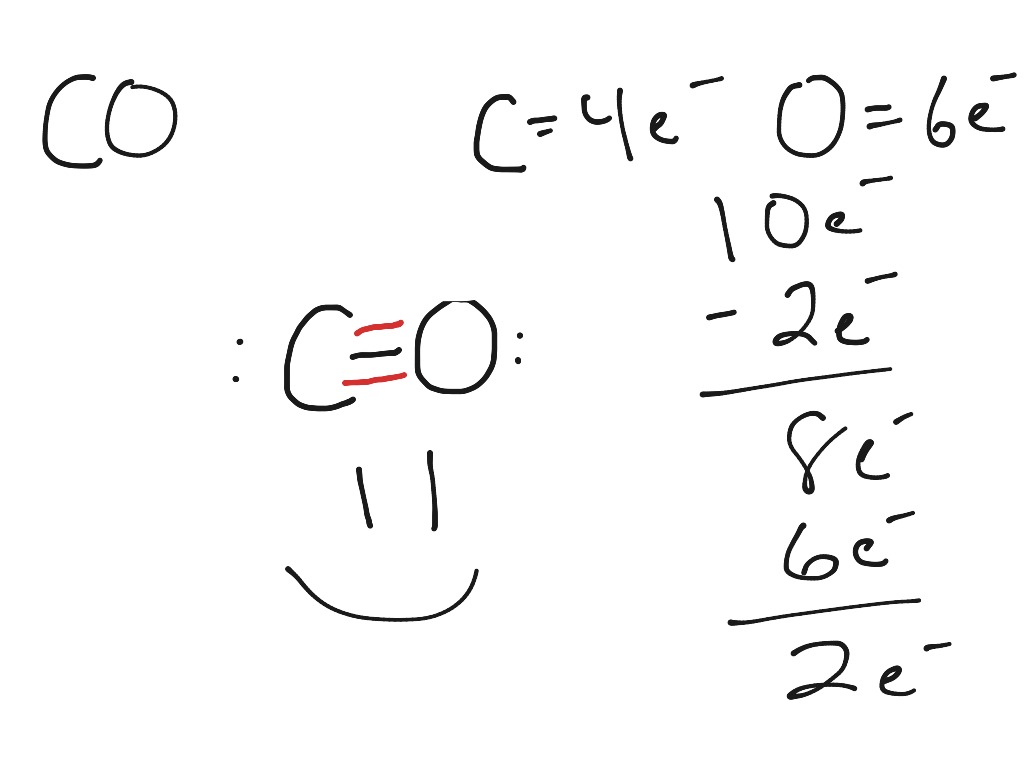
Lewis dot structure of carbon monoxide Science, Chemistry ShowMe
What is the Lewis dot structure for carbon monoxide? Chemistry Covalent Bonds Drawing Lewis Structures 1 Answer Anthony R. May 22, 2017 See explanation: Explanation: Begin finding the chemical formula for carbon monoxide. That is CO Then determine how many valence electrons each element has: Carbon has 4 valence electrons and Oxygen has 6.
What is the oxidation number of carbon monoxide? Socratic
Carbon monoxide is an odorless, colorless gas. It is produced any time a fossil fuel is burned and it can cause sudden illness and death. The Centers for Disease Control works with national, state.
Carbon Monosulfide Lewis Structure Molecule Carbon Monoxide, PNG
Hello Guys!In this video, we are going to look at the Lewis structure of Carbon Monoxide (CO).Carbon Monoxide is a diatomic molecule and is known for its pro.

Lewis dot structure of Carbon monoxide Drawception
The Lewis structure for CO (carbon monoxide) is a crucial component of understanding its molecular properties and reactivity. In this article, we will explore how to draw the Lewis structure for CO, and the significance of CO in various fields of chemistry. How to Draw the Lewis Structure for CO
Solved Part 1 Carbon Monoxide (A) Draw the Lewis structure
Watch on See the Big List of Lewis Structures Transcript: This is the CO Lewis structure: Carbon monoxide. We have 4 valence electrons for Carbon and 6 for Oxygen, for a total of 10 valence electrons. So we have a Carbon and an Oxygen atom bonded together.
Carbon monoxide. The two concentric semicircles in the Lewis structure
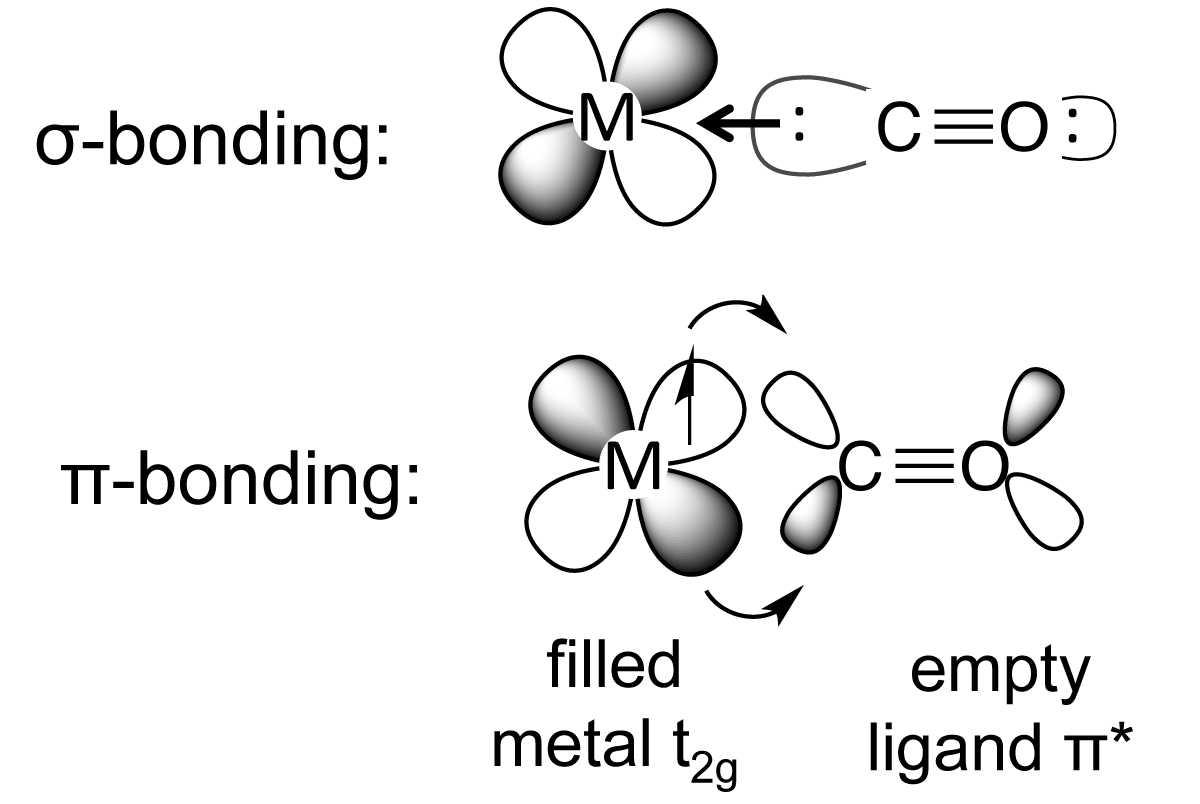
CO C O is usually considered a Lewis base, as the lone pair on carbon readily donates it's electrons, for example forming transition metal coordination complexes. Although oxygen is more electronegative than carbon, the carbon is more rich in electron density in the CO C O molecule as it basically has a full formal negative charge.

CO (Carbon Monoxide) Lewis Dot Structure with Formal Charge
Structural formula of carbon monoxide with three bonds and one lone pair on both carbon and oxygen. Carbon has a negative formal charge and oxygen has a positive formal charge. Notice that overall the carbon monoxide molecule is neutral. Oxygen has a plus charge and carbon has a minus charge. These charges cancel to give an overall neutral.

Solved The Lewis structure for carbon monoxide is C = O
A step-by-step explanation of how to draw the CO Lewis Dot Structure (Carbon monoxide ).For the CO structure use the periodic table to find the total number.
